
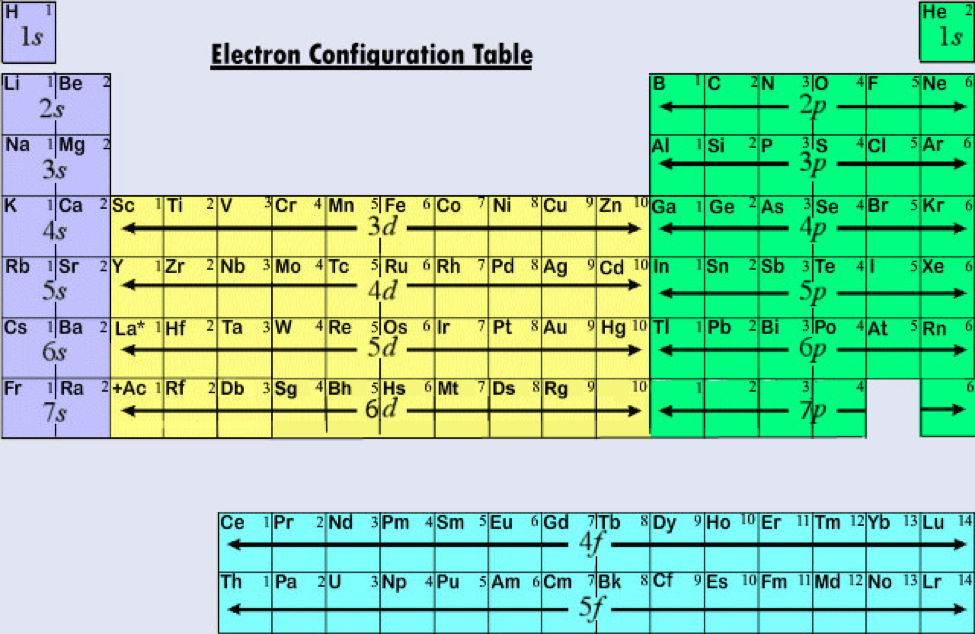
We will look at several visualizations of the periodic table. The shell model, as with any scientific model, is less a description of the world than a simplified way of looking at it that helps us to understand and correlate diverse phenomena.

In order to relate the properties of the elements to their locations in the periodic table, it is often convenient to make use of a simplified view of the atom in which the nucleus is surrounded by one or more concentric spherical "shells," each of which consists of the highest-principal quantum number orbitals that contain at least one electron these are s- and p-orbitals and can include d- or f-orbitals, which is atom dependent. The properties of an atom depend ultimately on the number of electrons in the various orbitals, and on the nuclear charge which determines the compactness of the orbitals. Each shell consists of one or more subshells, and each subshell consists of one or more atomic orbitals. The electrons in the partially filled outermost shell (or shells) determine the chemical properties of the atom it is called the valence shell. It therefore provides a useful framework for analyzing chemical behavior and is widely used in chemistry and other sciences. Using periodic trends, the periodic table can help predict the properties of various elements and the relations between properties. The f-block is usually not included in the main table, but rather is floated below, as an inline f-block would often make the table impractically wide. There are four distinct rectangular areas or blocks. Elements with the same number of valence electrons are kept together in groups, such as the halogens and the noble gases. The main body of the table is a 18 × 7 grid. Elements are presented in increasing atomic number. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. orbitalA specification of the energy and probability density of an electron at any point in an atom or molecule.electron shellThe collective states of all electrons in an atom having the same principal quantum number (visualized as an orbit in which the electrons move).quantum numberOne of certain integers or half-integers that specify the state of a quantum mechanical system (such as an electron in an atom).The electrons in the outermost or valence shell are especially important because they can engage in the sharing and exchange that is responsible for chemical reactions.The properties of an atom relate directly to the number of electrons in various orbitals, and the periodic table is much like a road map among those orbitals such that chemical properties can be deduced by the position of an element on the table.Elements are organized by period and group, with the period corresponding to the principle energy level, and the group relating to the extent the subshells are filled.


 0 kommentar(er)
0 kommentar(er)
